
AstraZeneca is one of the world’s leading pharmaceutical companies, producing a powerful range of medicines designed to fight disease in important areas of medical need. AstraZeneca was formed in April 1999 with the merger of Astra AB (an international pharmaceutical group based in Sweden) and Zeneca Group plc, a bioscience business, which itself was formed by a demerger from ICI in 1993. This merger brought together two companies with similar research-based cultures and a shared vision of the future of the pharmaceutical industry.
AstraZeneca now employs some 50,000 people worldwide and is a major player in the ethical pharmaceutical market, producing some of the world’s best known medicines. In 1999, sales totalled over £9bn, with an operating profit of £2.2bn. AstraZeneca has over 30 production sites in 19 countries and sells medicines in over 100 countries. It is truly a global business.
The merger of the two companies brought some important benefits. With 100 years of combined experience, AstraZeneca has been able to concentrate skills and resources to improve the performance of the business. There were also considerable savings to be made in production, sales and marketing and administration.
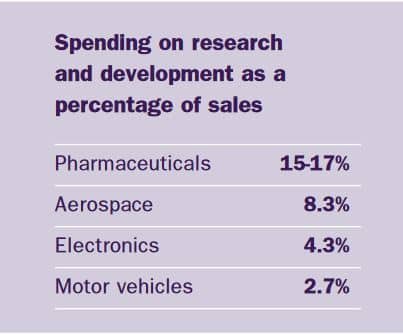
These cost savings resulting from a merger are called economies of scale or synergies. The range of products that the two companies produced complemented each other and, although there were areas in which both companies were working, the overlap was minimal. The result is one of the broadest, strongest and most exciting product portfolios in the industry. AstraZeneca produces seven products that each have annual sales of over £300m.
AstraZeneca produces pharmaceutical products in seven key areas:-
- Cancer
- Cardiovascular disease
- Central nervous system disease
- Gastrointestinal disease
- Infection
- Pain control and anaesthesia
- Respiratory disease.
AstraZeneca aims to become the world leader in at least four of these categories within the next ten years. In the UK, AstraZeneca is already a leader in three – gastrointestinal, cancer, anaesthesia – and second in two – respiratory and cardiovascular.
This case study examines the benefits of the merger, particularly in the field of research and development, and the challenges of marketing pharmaceutical products in a global market.
Research and Development of new treatments

One of the most important benefits of the merger was the opportunity to invest in AstraZeneca’s research and development programme.
By bringing together the capabilities and facilities, AstraZeneca has been able to dramatically increase the scale of the research and development, providing more manpower, improved, shared technology and a larger number of laboratories. The combined expertise of some of the world’s most talented scientists provides an exciting environment for discovery and development.
AstraZeneca is now the second largest investor in research and development in the pharmaceutical industry worldwide. The amounts spent are enormous. A total of £1.5bn was spent in 1999 alone, over £5m per working day worldwide. The UK has a fine reputation in the field of medical research and development; six of the world’s top 25 selling medicines were developed in British laboratories.
The merger has enabled AstraZeneca to increase investment in the discovery and development of new chemical compounds the key building blocks of new medicines, which could become major brands of the future. Owing to the highly complex nature of the products and the technology needed to develop them, the pharmaceutical industry spends more on research and development than other industries.
Product life cycle
As in many industries, AstraZeneca develops and manufactures products that have a natural life span. After a long period of development and testing, sales of the new medicine will hopefully increase before reaching a period of stability. Eventually, sales will fall as patent protection is lost and new advances in science lead to the discovery of more effective products which will in their turn come onto the market. This is known as the product life cycle.
Product portfolio
In the highly competitive pharmaceutical industry, it is essential for AstraZeneca to maintain a product portfolio at the cutting edge of modern medical science. Pharmaceutical companies need to ensure that they always have new products coming through the research and development process to replace older medicines and maintain a competitive, profitable product portfolio.
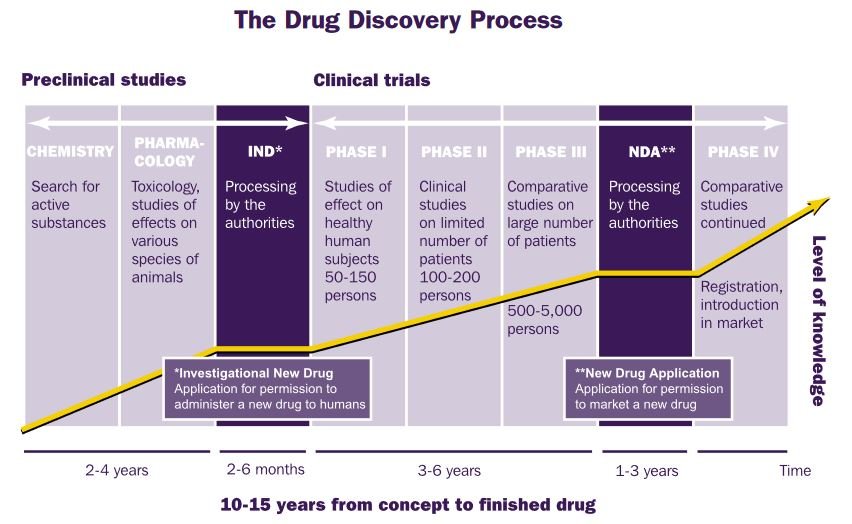
A company that stays still will not survive for long. Developing a new medicine requires a major commitment of time and resources. The path from the discovery of potentially effective medicine to its launch on the market is a lengthy and complex process and can cost some£500m. At AstraZeneca, this is called ‘The Development Pipeline’.
AstraZeneca has a significant number of products in the development stages. At any one time, the pipeline will have around 50 projects with many more to follow. At the start of 2000, there were 159 research and development projects, including 57 brand new compounds in the pipeline.
The ambitious targets for AstraZeneca’s research and development teams are to deliver 12 new compounds by the end of 2001 and thereafter 15 new ones every year by the year 2003. The company’s objective is to double the value of its pharmaceutical product portfolio every five years.
It takes approximately 10 to 12 years to bring a brand new drug to the market. Initial laboratory work might identify a new chemical compound that is promising. The structure will be further refined and developed until it is ready for clinical trials. Only a small number of compounds successfully make it to this stage.
Bringing a product to market
Trials take place in three phases.
- Phase I involves the drug being given to 50-150 healthy volunteers to see how it works in humans and help establish the correct dosage. This phase lasts for approximately two years.
- Phase II also extends for two years and tests the drug on a small, probably 100 – 200, number of patients with the condition that is being targeted. The medicine is assessed to determine the dose at which it works and that is does not produce any unacceptable side effects.
- Phase III, the final period of trials extends this to between 500 and 5000 patients and lasts for three years. These clinical trials are absolutely essential to guarantee that the medicine works and that there are no potentially serious side effects.
Providing the safety and effectiveness of the drug can be established by the results of the trials, AstraZeneca can apply to the national licensing authorities for permission to market the drug. Even then, close monitoring of the drug’s performance will be necessary to ensure there are no adverse effects.
The key to AstraZeneca’s programme is the ability to select those compounds with the greatest potential to become significant advances in healthcare in the future. Across the pharmaceutical industry, only one in ten candidate drugs will actually make it through the process and on to the market.
No pharmaceutical company would invest £500m in research and development if, as soon as the drug was released onto the market, all its competitors were able to analyse the compounds in their own laboratories, copy them and release their own branded versions at a fraction of the cost of development. It is essential that all pharmaceutical companies are able to recoup their investment in time and money over a period of time following the drug’s release onto the market. This protection is achieved through the use of patents.
What is a patent?
Rather than try to keep a new compound secret, which is virtually impossible, inventors apply for a patent. This is a form of legal protection that prevents illegal copying. These statutory rights will last for a set period, usually 20 years, after which other manufacturers are free to produce their own, possibly cheaper versions of the drug called generics.
Pharmaceutical companies can apply for patents of the molecules of the new compounds at a very early stage of the research and development process to prevent new discoveries from leaking out. Thereafter the company can apply for further patents to protect the development process, the formulation process and even the shape of the tablet or capsule.
Research into new medicines would be unthinkable in an environment without this protection as the company would be unable to recover the original research and development costs. This is an example of the legal system protecting and helping the manufacturer.
Global marketing of new drugs
Having acquired a licence, AstraZeneca has to market its new drug and attempt to earn a return on the investment. This is by no means an easy or foregone conclusion. Most new products face stiff competition from established medicines and doctors may need to be convinced to prescribe a brand new product in place of a tried and tested medicine.
Although a patent may last 20 years, the new drug may have been ten years in development. This drastically reduces the time available to recoup the cost of research and development. Further competition may come from other products, different in their make-up, but launched in the same drug class to treat the same illnesses.
All these influences make the marketing of pharmaceutical products intensely competitive. Consequently, marketing expenditure can be extremely high and AstraZeneca employs over 24,000 people in sales and marketing worldwide. However, the rewards for a successful brand can be considerable. By way of comparison, annual sales of Snickers, the world’s largest selling chocolate bar are $1bn. AstraZeneca’s Losec® – the world’s number one selling prescription medicine – has annual sales of $6bn.
The best way to ensure financial success is to discover and market a drug substance that treats an illness in a way that no existing medicine has done before. AstraZeneca employs some 10,000 people in the UK, who work in all areas of the business including Research & Development, Production and Sales and Marketing.
Sales and Marketing have 1,800 of these employees, the majority of whom work in the sales field, visiting GP surgeries, hospital consultants, nurses and NHS budget decision-makers. They are provided with all the necessary promotional materials and information about prescribing the new medicine.

Conclusion
Marketing a new medicine globally can often present particular challenges, as patent restrictions can vary from one country to another. Systems of healthcare vary enormously and different countries have different restrictions on the ways that medicines can be promoted.
For example, in Australia, the National Health System means that patients have to pay a fee to visit their doctor, whilst in the UK these visits are free. In the USA, AstraZeneca can advertise prescription medicines directly to the public, but this is not allowed in the UK. This will reduce awareness of the brand amongst consumers and will need different marketing approaches.
AstraZeneca plans to build on the position of strength that has been created by the merger, using the best of each of the partners.
